Recombinant therapeutic proteins are biologic drugs produced by bioengineered bacteria or cultured cells, the host cells. Over 100 recombinant proteins have been approved by the FDA and many more are being tested. Typically an ELISA assay is used to quantify host cell protein (HCP) contamination of the product. The 1D and 2D SDS PAGE tests described on this page are used to characterize anti-HCP antibodies chosen for the ELISA, and to monitor Drug Substance purification steps.
1D HCP Antibody Screening: Anti-HCP antibodies are polyclonal, usually from rabbits or goats, because the antigenic protein mixture is complex. 1D western blotting is a good way to check the time course of HCP antibody production and to compare reactions of different animals. This can serve as a preliminary check to ensure the best antibody or combination of antibodies is used for developing the assay.
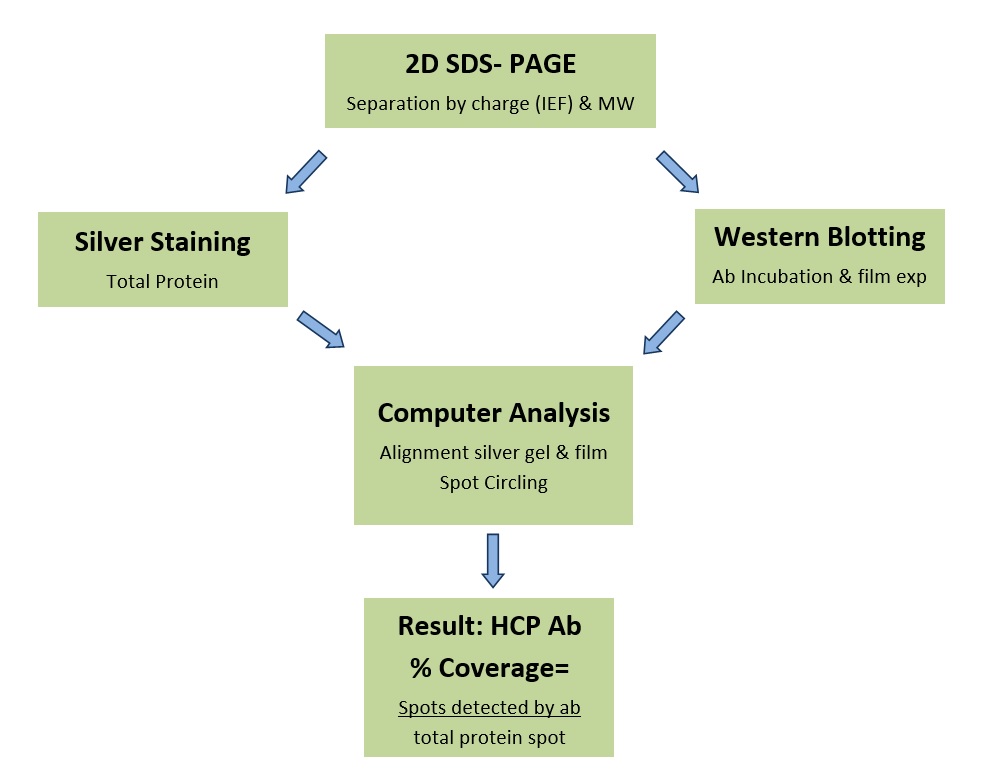
HCP Coverage Testing: In this test, outlined in the schematic below, the number of protein spots on 2D film patterns from an optimized HCP western blot obtained with the antibody in question are compared to the number on duplicate silver-stained patterns. Light and dark films are used to create a detailed report of the antibody coverage (% of total proteins detected by the antibody). Our white paper gives an overview of coverage testing. Follow the links for more information about method, results, and pricing.
DS Testing: HCP contaminants are sometimes similar in molecular weight and isoelectric point to the DS and thus difficult to detect by 1D SDS PAGE and mass spectrometry. Orthogonal evidence confirming that the DS contains few or no detectable HCP proteins is desirable. 2D-SDS PAGE, an orthogonal test, may be used to follow HCP removal during various processing steps of a purified Drug Substance (DS) and to check the final product (see DS/In-Process Testing or Product Testing for more information).
The PowerPoint HCP Antibody Analysis (pdf) provides more information about host cells, biologic drugs and uses of 2DE (2D Electrophoresis) in recombinant protein drug production. Quality assurance monitoring is in place for 1D and 2D-SDS PAGE at Kendrick Labs including >100 SOPs, documented employee training, and QA review of all reports. Run documentation and tracking (PDF) is recommended to retrace the procedures that were followed.