Lysine acetylation is an important regulatory factor for metabolic enzyme activity in both bacteria (Salmonella enterica) and mammalian liver (1-3). This post-translational modification has also been implicated in sirtuin modulation of longevity (4) and the mammalian liver response to alcoholism (5). It appears that dynamic protein acetylation might be as widespread and important a PTM as phosphorylation (1).
We now offer Western blotting (WB) of acetyl-lysine residues on proteins resolved by 2D electrophoresis as shown in Figures 1 and 2 below.
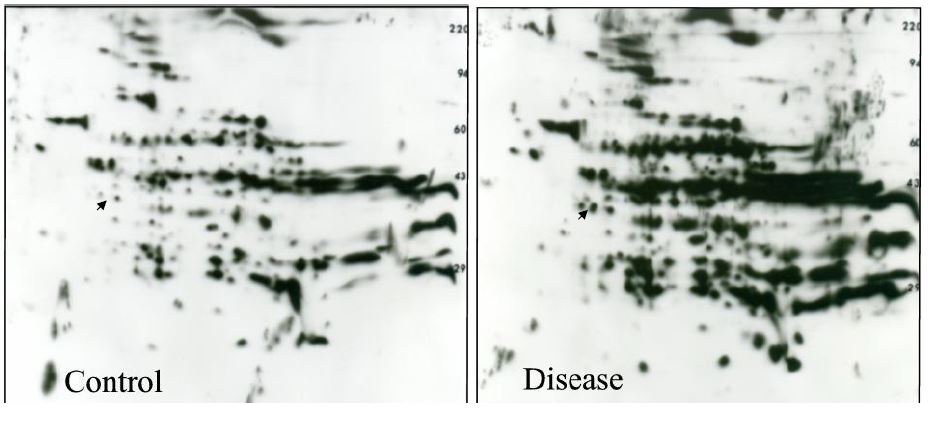
Figure 1. 2D ECL films showing increased lysine acetylation in rat liver associated with a disease state. 2D gels were loaded with equal amounts of protein from control and disease liver cytosol. An anti-acetylated-lysine antibody from Cell Signaling was used for WB at a 1:1000 dilution with overnight incubation. The images are shown with permission of Blythe Shepard and Dr. Pamela Tuma, Catholic University of America, Washington, DC. See reference 5 for more information.
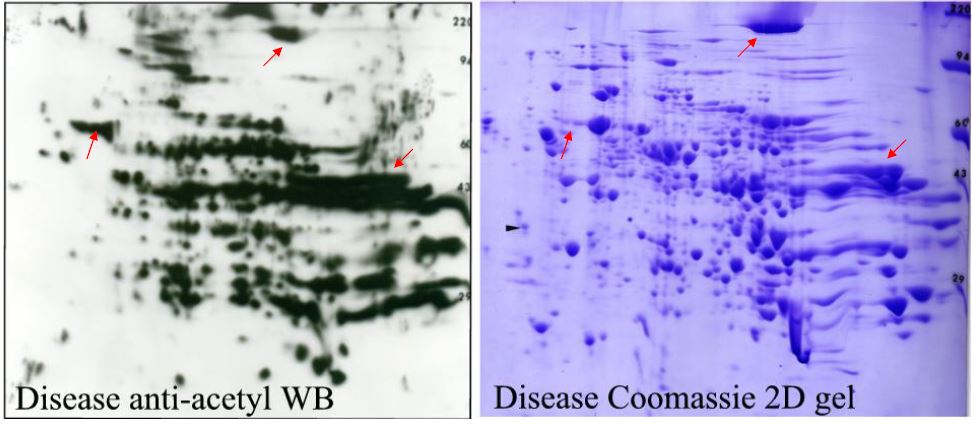
Figure 2. Matching between ECL film and duplicate Coomassie gel. Each ECL film is superimposable with an image of the stained blot. Protein spots of interest found on the image are easily matched to a duplicate Coomassie blue stained gel for spot-cutting. Any CB spot, no matter how faint, is within range for identification by MS.
2D electrophoresis, acetyl-lysine western blotting and subsequent mass spectrometry allow identification of specific acetylated proteins changing between samples.
Acetyl-Lysine Western Blotting (BP-10) Package includes: 2D gel electrophoresis, transblotting to PVDF, blot Coomassie staining, subsequent immunostaining with anti-acetyl-lysine antibody (Cell Signaling Technologies’ polyclonal anti-acetyl-lysine antibody), and electronic photos of stained blot and ECL film. Options for additional services including duplicate Western blots, and mass spectrometry are also available. Follow the link for pricing.
References
1. Norvell, A. and S.B. McMahon, Perspectives Cell biology: Rise of the rival. Science, 2010 327(5968): p964-5.
2. Zhao, S., W. Xu, W. Jiang, W. Yu, Y. Lin, T. Zhang, J. Yao, L. Zhou, Y. Zeng, H. Li, Y. Li, J. Shi, W. An, S.M. Hancock, F. He, L. Qin, J. Chin, P. Yang, X. Chen, Q. Lei, Y. Xiong and K.L. Guan, Regulation of cellular metabolism by protein lysine acetylation. Science, 2010. 327(5968): p. 1000-4.
3. Wang, Q., Y. Zhang, C. Yang, H. Xiong, Y. Lin, J. Yao, H. Li, L. Xie, W. Zhao, Y. Yao, Z.B. Ning, R. Zeng, Y. Xiong, K.L. Guan, S. Zhao and G.P. Zhao, Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science, 2010. 327(5968): p. 1004-7.
4. Lombard, D.B., F.W. Alt, H.L. Cheng, J. Bunkenborg, R.S. Streeper, R. Mostoslavsky, J. Kim, G. Yancopoulos, D. Valenzuela, A. Murphy, Y. Yang, Y. Chen, M.D. Hirschey, R.T. Bronson, M. Haigis, L.P. Guarente, R.V. Farese, Jr., S. Weissman, E. Verdin and B. Schwer, Mammalian sir2 homolog sirt3 regulates global mitochondrial lysine acetylation. Mol Cell Biol, 2007. 27(24): p. 8807-14.
5. Shepard, B.D., D.J. Tuma and P.L. Tuma, Chronic ethanol consumption induces global hepatic protein hyperacetylation. Alcohol Clin Exp Res, 2010. 34(2): p. 280-91.