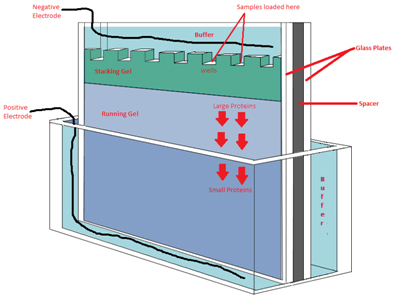
Schematic representation of an
electrophoresis gel.
1D Electrophoresis is a method that separates protein by molecular weight over a range of about 10 to 300 kilodaltons (kDa). In this method, samples are weighed and dissolved in sodium dodecyl sulfate (SDS). SDS is a negatively charged detergent that has both hydrophilic (likes to associate with water) and hydrophobic regions (does not like to associate with water). SDS likes to bind to proteins with its hydrophobic region and also likes to be in water in its hydrophilic region. This SDS- protein-water interaction allows water insoluble proteins to dissolve in water, making SDS the best detergent to dissolve protein mixtures. The proteins are completely denatured (unraveled from their folded native structure) by the SDS. When an electric field is applied, the negative charge of the SDS causes the proteins to move through a clear acrylamide matrix toward the positive electrode. This matrix has holes in it that sieve out the proteins by molecular weight. Large proteins move more slowly through the matrix than the smaller proteins thereby separating proteins by molecular weight. Molecular weight standards are included on each gel to allow for determination of protein size.