Optimization of pSer and pThr Western Blotting.
Why do it? It is now known that the human genome has a remarkable 518 protein kinases (1). The kinases act to dynamically phosphorylate 30 – 70% of mammalian cellular proteins on amino acids serine, threonine and tyrosine with an approximate ratio of 1800: 200: 1 respectively (2, 3). Phosphorylated proteins mediate cell division, cellular differentiation, hormonal signal transduction and much more. While tyrosine phosphorylation often initiates major cellular events, phosphorylation of serine and threonine residues on proteins are key to the cascades of reactions that follow and are of great interest to many clients.
In the past, results have been poor for PSer and PThr Western blotting. Because the epitopes are small and the antibodies weak, a low dilution was required, from 1:200 to 1:500. A dark, blotchy background often came up because the film exposure time was too long, about 20 min. The proteins that lit up often match to the major proteins on the corresponding 2D gel, suggesting nonspecific binding. Because the antibodies were expensive, and couldn’t be diluted out, we couldn’t do our usual free repeats if the blots showed problems. Eventually we just stopped offering this service.
However, a new product, ECL Advance from GE Healthcare, greatly increased the sensitivity of the method by emitting more light. We reasoned that with the increased sensitivity we could dilute the antibodies 1:5000 instead of 1:200. Nonspecific binding should be less pronounced; the Western blots should have more meaning; the ab cost per gel would be greatly reduced. So we spent considerable time optimizing conditions for the new ECL Advance reagent. We chose the Qiagen antibody Q5 (IgG & IgM) for detection of phosphorylated serine residues and Q7 (IgG) for detection of phosphorylated threonine residues because both were advertised by Qiagen as being general antibodies with binding that were independent of surrounding amino acids. At least 5 publications (4-8) describe use of these antibodies for Western blotting.
After about ten 1D and 2D gel optimization experiments, we started getting the following results:
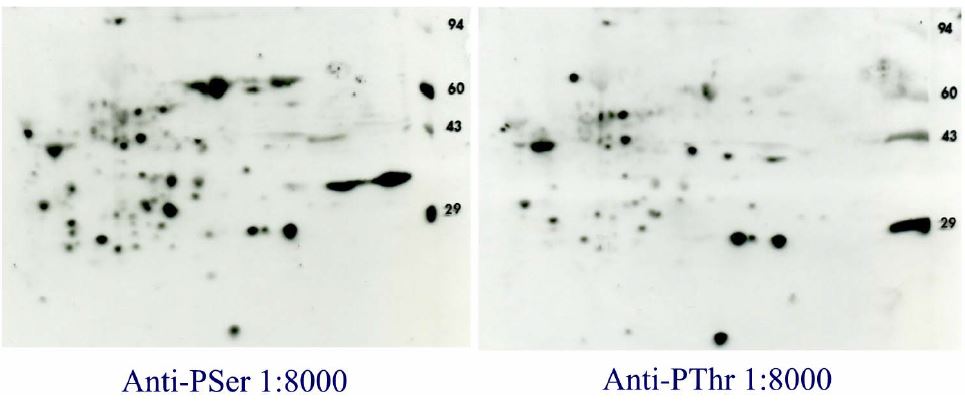
Qiagen Q5 (Anti-PSerine) versus Q7 (Anti-PThreonine) Western blot results with ECL Advance for 2D gels run with rat liver homogenate (RLH). The same RLH sample (200 ug) was loaded on two 2D gels run identically at Kendrick Labs followed by identical Western blotting except for the primary antibody. Even though the antibodies were diluted 40 times more than recommended for ECL (1:200), they gave a better pattern than any previously seen. Some proteins are common to both 2D gel films as might be expected, but the patterns do not match. Secondary antibody alone gives an essentially blank film. Conclusion: ECL Advance does greatly increase sensitivity for phosphoprotein Western blotting.
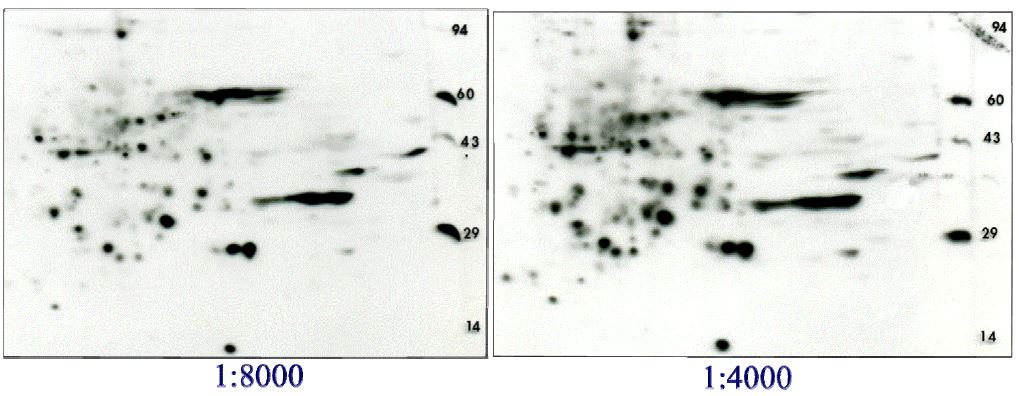
Most people want us to combine the PSer and PThr antibodies and do a single Western blot to save cost and precious sample. The figure below shows the results of the combined antibodies at 1:8000 versus 1:4000 dilution, still 20 times the recommended amount.
Western blot results for combined Q5 and Q7 antibodies. Again, 200 ug of rat liver homogenate was run identically on two 2D gels. The antibodies were combined before the dilutions and subsequent Western blotting with ECL Advance. The lower dilution of 1:4000 clearly gives a darker yet clean pattern which is otherwise identical to the 1:8000 dilution. The combined antibodies at 1:4000 dilution is currently offered in the packages below.
For a published example, see Figure 6 in the paper: Liu, X., et. al. “Disruption of Striated Preferentially Expressed Gene Locus Leads to Dilated Cardiomyopathy in Mice” Circulation, 2009. 119(2): p. 261-8.
Follow the link to see details and packages prices are available for combined (BP-8) P-Serine/P-Threonine or individual (BP-9) Western blotting.
References
1. Manning, G., Whyte, D.B., Martinez, R., Hunter, T. and Sudarsanam, S. The Protein Kinase Complement of the Human Genome, Science 2002, 298: 1912-1934.
2. Mann, M., S.E. Ong, M. Gronborg, H. Steen, O.N. Jensen and A. Pandey, Analysis of protein phosphorylation using mass spectrometry: Deciphering the phosphoproteome. Trends Biotechnol, 2002. 20(6): p. 261-8.
3. Olsen, J.V., M. Vermeulen, A. Santamaria, C. Kumar, M.L. Miller, L.J. Jensen, F. Gnad, J. Cox, T.S. Jensen, E.A. Nigg, S. Brunak, and M. Mann, Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Science signaling, 2010. 3(104): p. ra3.
4. Daquinag, A., M. Fadri, S.Y. Jung, J. Qin and J. Kunz, The yeast ph domain proteins slm1 and slm2 are targets of sphingolipid signaling during the response to heat stress 10.1128/mcb.00461-06. Mol. Cell. Biol., 2007. 27(2): p. 633-650.
5. Jablonowski, D., L. Fichtner, M.J.R. Stark and R. Schaffrath, The yeast elongator histone acetylase requires sit4-dependent dephosphorylation for toxin-target capacity 10.1091/mbc.E03-10-0750. Mol. Biol. Cell, 2004. 15(3): p. 1459-1469.
6. Kalabis, J., I. Rosenberg and D.K. Podolsky, Vangl1 protein acts as a downstream effector of intestinal trefoil factor (itf)/tff3 signaling and regulates wound healing of intestinal epithelium 10.1074/jbc.M512905200. J. Biol. Chem., 2006. 281(10): p. 6434-6441.
7. Lamkemeyer, T., B. Zeis, H. Decker, E. Jaenicke, D. Waschbusch, W. Gebauer, J. Markl, U. Meissner, M. Rousselot, F. Zal, G.J. Nicholson and R.J. Paul, Molecular mass of macromolecules and subunits and the quaternary structure of hemoglobin from the microcrustacean daphnia magna. FEBS J., 2006. 273(14): p. 3393-3410.
8. Mulet, J.M., D.E. Martin, R. Loewith and M.N. Hall, Mutual antagonism of target of rapamycin and calcineurin signaling 10.1074/jbc.M604244200. J. Biol. Chem., 2006. 281(44): p. 33000-33007.