Optimization
Optimization of Phosphotyrosine 2D Western Blots
Tyrosine phosphorylation is an important post-translational modification involved in many aspects of cell growth and differentiation. For example, non-proliferating cells have very low levels of tyrosyl phosphorylated proteins while dividing cells have activated tyrosine kinases (TK) that generate relatively high levels of phosphorylated species (1). TK oncogenes have been shown to drive cell division during various kinds of cancer (2); many kinase inhibitors are undergoing clinical trials. ELISA and kinase arrays exist to measure tyrosine kinases but there are few ways to measure unknown TK substrates. Thus we decided to optimize phosphotyrosine (PTyr) Western blotting and provide it as a service.
Examples of 2D Phosphotyrosine Western Blots
Initially, the specificity of the antibody was tested with a recombinant tyrosine kinase standard, VEGF-R. The schematic shown in Figure 1 gives an explanation of the mechanism of action of VEGFR; Figure 2 shows the results of our Western blots (WB).
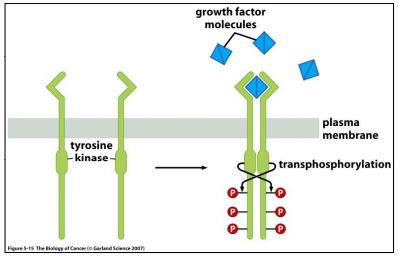 |
| Figure 1. Tyrosine Kinase Receptors dimerize following ligand binding, and transphosphorylate tyrosines on the cytosolic chains. Taken from “The Biology of Cancer” by R Weinberg, p137. |
| VEGF, a 38.2 kDa homodimeric protein, stimulates the formation of a new blood supply for developing tumors by triggering dimerization and transphosphorylation of its receptor as shown above. We bought recombinant VEGF-R2 from ProQinase, 32P labeled 1 μg assuming self-phosphorylation, TCA precipitated to concentrate, ran a 2D gel, transferred to PVDF, exposed 4 days and then did an anti-PTyr Western blot. Results are shown in Figure 2 below. |
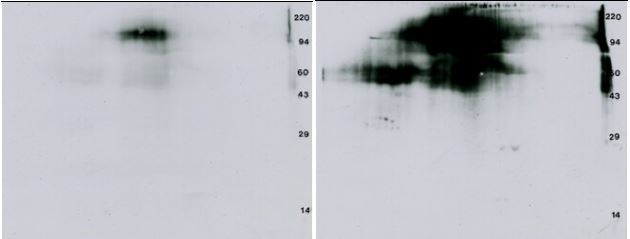 |
| Figure 2, Left: 32P Autorad: 4 day x-ray film exposure to show 32P labeled VEGF-Receptor. Right: Western blot ECL film: 30 second film exposure after immunostaining the blot on the left with the PY20 anti-PTyr antibody. |
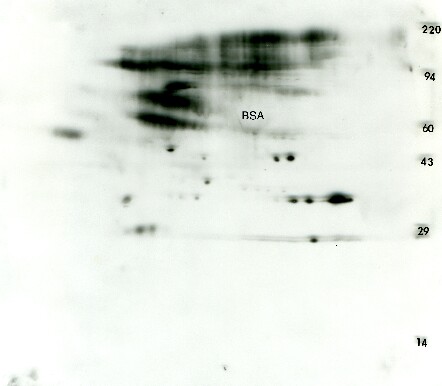
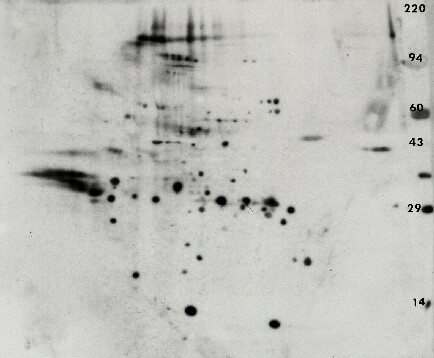
Figure 3. Anti-phosphotyrosine Western blot from transformed 3T3 cells. A standard format 2D gel was run with just 25 μg of transformed 3T3 cells and Western blotted with the Exalpha PY20 antibody. The ECL detection kit from GE Healthcare was used with x-ray film to visualize the pattern. The ECL film shows about 10 putative glycosylated proteins characterized by multiple fuzzy isoforms along with 12 proteins showing single, double or triple charge isoforms that would traditionally be expected from phosphorylation. The glycosylated, phosphorylated proteins are probably membrane receptors.Only one of the ECL spots could be matched to a faint protein on a Coomassie blue stained duplicate gel (not shown) suggesting that this antibody is quite sensitive. The large presumptive bovine serum albumin (BSA) protein spot from the culture media is negatively staining.
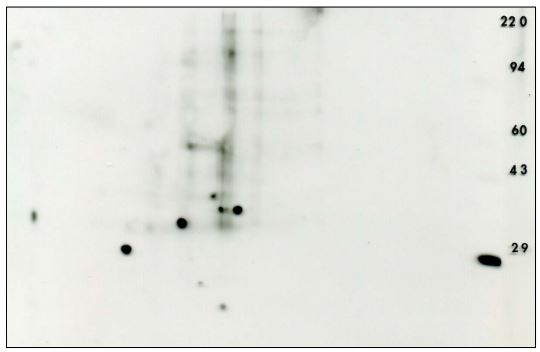
Figure 4. Anti-phosphotyrosine 2D Western blot from an erythroid progenitors sample. The PVDF blot was immunostained with the Exalpha PY20 antibody under standardized conditions. The ECL detection kit from Pierce was used with x-ray film to visualize the pattern. A longer film exposure was performed to reveal more detail. Shown with permission of Dr. Amittha Wickrema, University of Chicago.
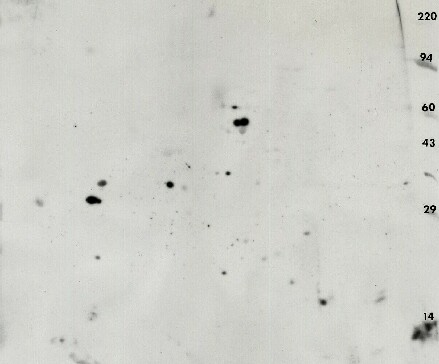
Figure 5. Anti-phosphotyrosine 2D Western blot using the PY20 antibody obtained from mouse embryonic fibroblast after siRNA knockdown The ECL detection kit from Pierce was used with x-ray film to visualize the pattern (shown with permission). The 29 thousand MW marker, carbonic anhydrase, is sold as a phosphotyrosine marker by Axxora.
Figure 6. Anti-phosphotyrosine 2D Western blot using the PY20 antibody against rat retinal pigment, eye epothelium. The ECL detection kit from Pierce was used with x-ray film to visualize the pattern.
1D Optimization showed the Exalpha antibody was the best value.
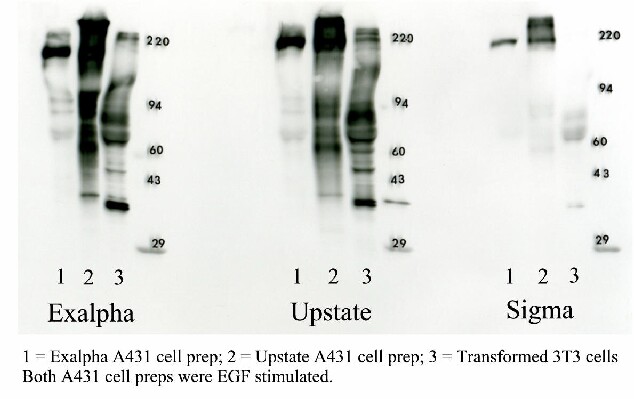
Three commercially available mouse monoclonal antibodies against phosphotyrosine were tested: 1. ExalphaBio Clone PY20, 2. Upstate Clone 4010, and 3. Sigma-Aldrich Clone PT-66. Figure 1 shows a comparison of the three antibodies under identical Western blotting conditions for three commercially available samples.
Figure 7. 1D optimization of phosphotyrosine Western blotting. The 3 Western blots shown above were obtained by running three standards in triplicate on a single gel in the lane order: sample 1 (EGF stimulated A431 cell prep purchased from Exalpha), sample 2 (EGF stimulated A431 cell prep purchased from Upstate), sample 3 (transformed 3T3 cell prep purchased from Exalpha), MW markers, blank lane. The gel was transblotted to PVDF, the blot stained with Coomassie blue and then divided into thirds through the blank lanes. Each third was Western blotted with a different antibody under identical conditions using ECL technology (GE Healthcare) to visualize results.
The PY20 antibody from Exalpha Biologicals gave an identical pattern to the 4010 clone from Upstate; both were superior to the pattern from the Sigma PT66 antibody. The Exalpha antibody is the best value and our choice for the package offered (see prices tab). Note that the 1D patterns from the same cultured cell line prepared by different companies were dramatically different (lanes 1 & 2) , suggesting that sample preparation is critical.
Discussion/References
Discussion and References
Genomic methods have many uses but don’t directly reveal what’s happening to cellular proteins during dynamic disease processes. For this the proteins themselves must be studied. Comparing complex 2D patterns of diseased to normal tissue is one way to study protein changes but the results will be confusing if cellular differentiation is involved. Numerous proteins from alternate differentiation pathways, for example, tumor versus adjacent normal liver sample, will show up as differences. A better approach is to focus on tyrosine kinase (TK) phosphorylation that activates known or novel cancer drivers to identify pathways that could be candidates for therapy or further studies.
We have optimized a method for detecting tyrosine phosphorylation using 2D Western blotting with an anti-phosphotyrosine antibody, PY20. Our results suggest that the method is specific, reproducible, and sensitive. A common problem, however, is that the proteins of interest will sometimes be in too low abundance to identify by mass spectrometry on a duplicate Coomassie blue stained gel.
References
1. D. Krause and R. Van Etten, NEJM , 353: 172-187, 2005, “Tyrosine Kinases as Targets for Cancer Therapy”
2. Robert A. Weinberg, “The Biology of Cancer”, 2nd Edition, Garland Science, New York, NY, 2013.
Packages
PHOSPHOTYROSINE WESTERN BLOTTING PACKAGE INCLUDING PY20 ANTIBODY.
Standard format (BP-7SF) and large format packages (BP-7LF) are available. These packages include: 2D gel electrophoresis, transblotting to PVDF, blot Coomassie staining, subsequent immunostaining with PY20 antibody, and electronic photos of stained blot and ECL film. Discounted pricing is available for duplicate blots.
Mass spectrometry to identify proteins and phosphorylation sites found by Western blotting is extra and requires a duplicate Coomassie blue stained gel.
Link for pricing for: PTyr WB package, Mass spectrometry, 2D gels
Ask for a quote by calling 800-462-3417 or emailing 2d@kendricklabs.com . Note that PTyr WB patterns may change after samples sit frozen for several weeks. Duplicate blots at the onset are better than retesting later.