This complex assay has been standardized at Kendrick Labs. SOPs are in place for every step; Biochemists and 2D Analysts are well trained.
2D SDS PAGE:
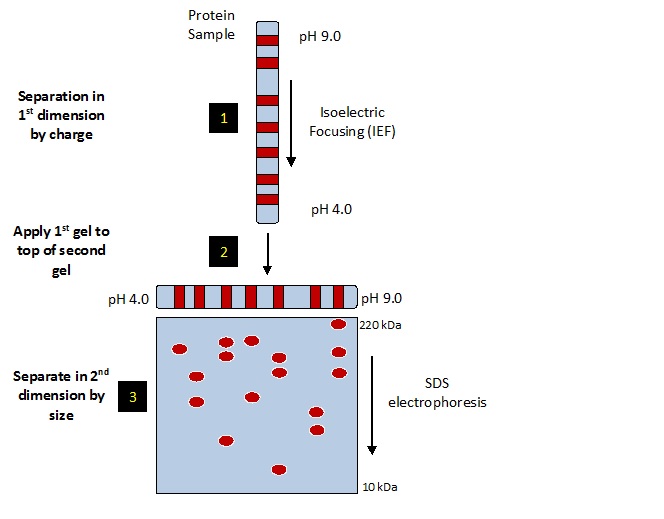
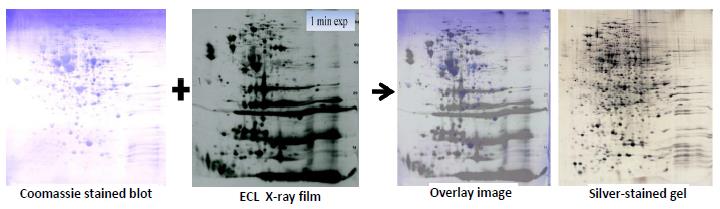
The sample is desalted and concentrated as needed. If the protein concentration of the sample is not known a BCA protein assay is done to determine protein amount. The sample is loaded on two silver stained gels and one PVDF membrane. The PVDF membrane is Coomassie blue stained and desktop scanned. The membrane is destained and western blotted with your antibody. We start with low antibody concentration and increase to give the best coverage with acceptable background. If the primary antibody is not HRP linked a secondary HRP linked anti primary antibody is used. The HRP linked antibody is detected with chemiluminescence using ECL reagent and X-ray film with multiple exposures. The 2DE method used is SDS compatible, see our whitepaper (PDF) for details.
The Western-Silver Alignment PPT illustrates how the complex silver-stain pattern is matched to that of the western blot film. For more information with references, see the white paper that accompanied our poster at the 2015 BEBPA HCP Workshop.
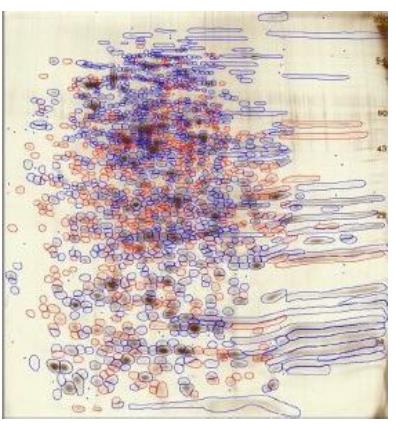
- Western silver alignment: Matched spots from silver-stained gels and western blots are used to align silver-stained gels and films.
- Spots present on silver-stained gel are circled in Progenesis software. Those detected only by antibody on western blot film are shown as small circles. Blue circled spots are on both film and silver gel. Red spots are only present on the silver-stained gel.
- Coverage is determined by # of protein spots detected by antibody (film) and total # of protein spots in sample (silver).
Analysis is highly reproducible between distinct analysts (5.1-8.1% difference).